Persistence Monitoring
Use Clear Safety™’s Similarity Analysis™ to discern resident from transient Listeria contamination for your in-plant operations.
PCR, culturing, and antigen-based tests tell you whether a pathogen is present or absent. Clear Safety™ is much more.
Clear Safety™ enables deep molecular characterization of pathogens, with just the amount of information needed for your safety program. You can choose to drill down to serotypes, strains, or whole genome sequences based on the application need.
Use Clear Safety™’s Similarity Analysis™ to discern resident from transient Listeria contamination for your in-plant operations.
Locating the source or supplier isn’t a problem since whole genome sequencing can be integrated into the platform.
We do serotyping at a fraction of the cost, and the turnaround time is significantly faster.
Clear Safety’s automation guarantees reproducibility and reduces hands-on labor and error potential.
Our automated workflow reduces hands-on time, technician error, and variability.
Clear Safety™’s accuracy sharply reduces false negatives and positives, curtailing recall risks, operational costs, holding time, and short-shipping penalties.
Our superior accuracy significantly outperforms even the best PCR detection kits in the market. We dramatically reduce the rate of false negatives or false positives, giving you the best screening assurance in the industry.
Eliminate Operational Inefficiencies Clear Safety reduces the number of false positives, enabling your lab to stop chasing ghosts and start shipping product. Contract service labs, in combination with Clear Safety’s serotyping feature, no longer need to wait days to confirm a true customer positive and not an internal control strain.
With Clear Safety, you can reduce costs, and the platform’s fast turnaround time keeps your inventory shipments moving.
Eliminate inventory hold time waiting for a positive confirmation. With Clear Safety, you can react on a presumptive with >99.9% accuracy.
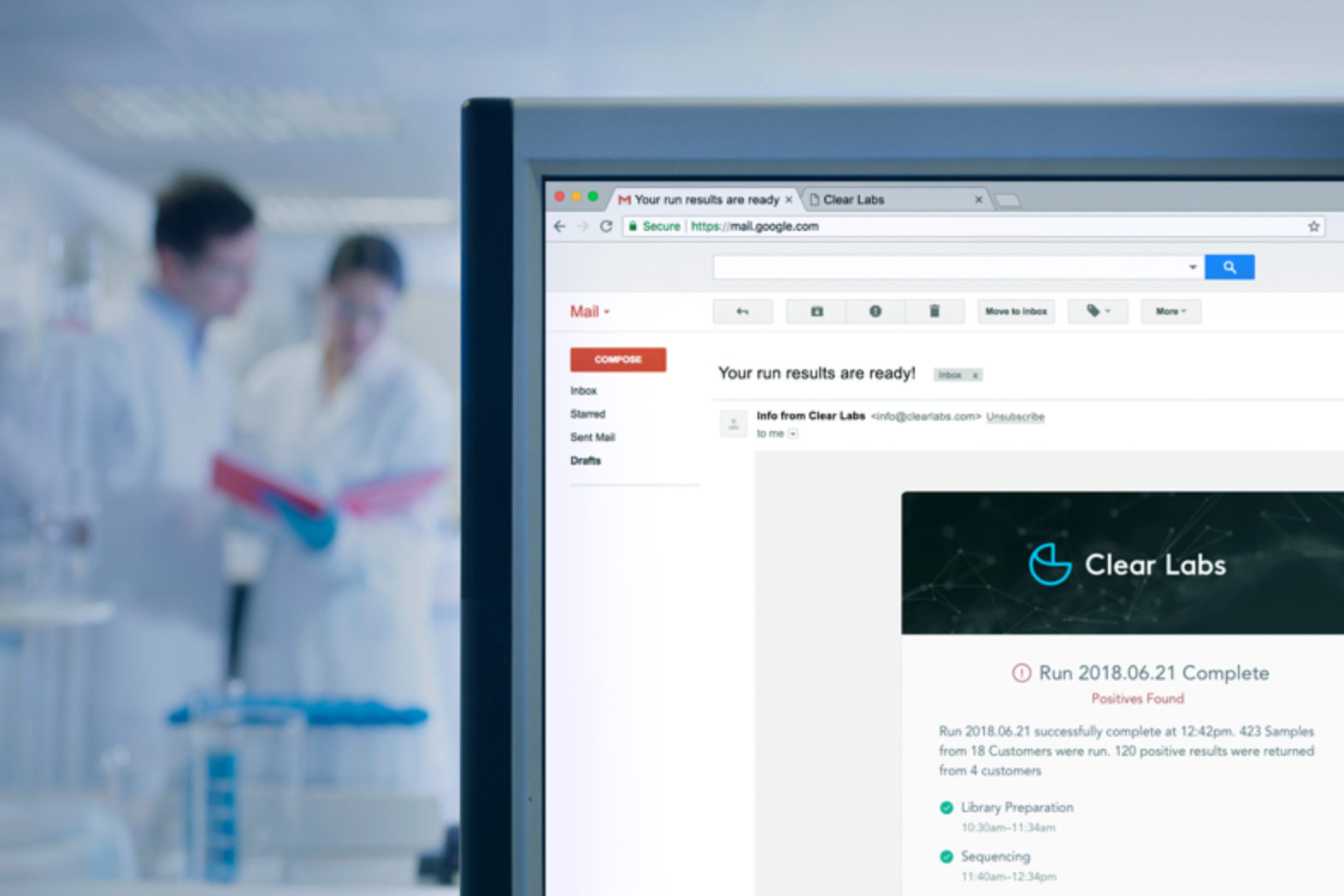
Within 24 hours, find out Salmonella serotype information and take action. With 36-hour results for Listeria, not only do you get screening results, but you also have access to persistence monitoring information and mapping visualization, both of which take several days to produce using legacy technology.
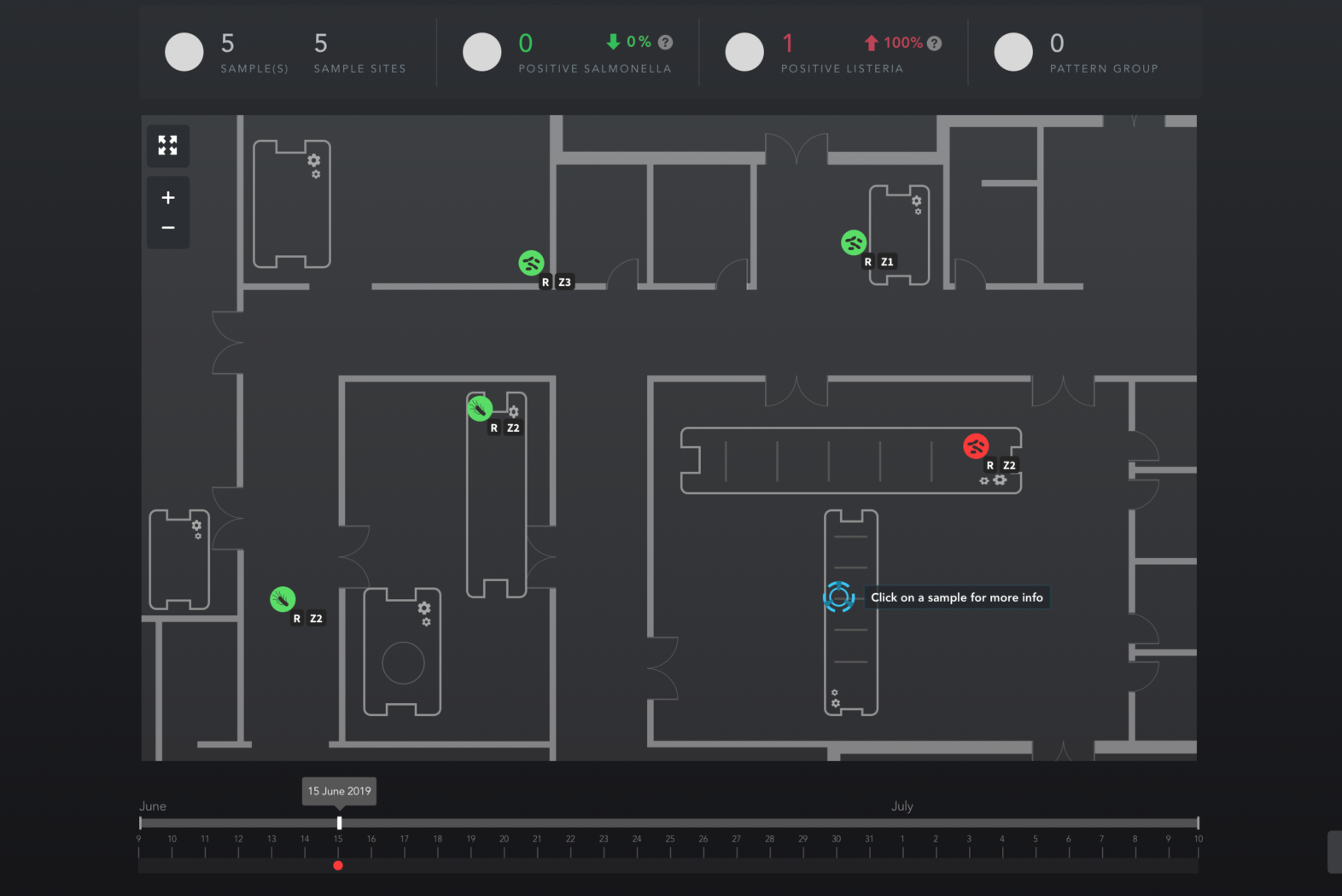
Visualize your environmental test results so that you can better understand contamination incidents and take more informed corrective actions.
Easily upload floor plans and choose sample sites for routine environmental testing.
See your test results on a map so that you can assess the state of your plant with one glance.
Determine which pathogen subtypes have appeared in your facility before. Then, look at historical data to track the movement of a particular subtype through your facility.